Rosenberg’s Research Spans a Half-Century and Counting
Cancer remains one of the principal causes of death among humans worldwide. According to the American Cancer Society in, 2024, total 1,806,590 new cases and 606,520 deaths have been reported due to cancer in the United States. Men report a higher incidence of prostate cancer, and women have a higher incidence of breast cancer. However, the highest number of deaths have been reported due to lung and bronchiolar cancer . The current therapeutic approaches include surgery, chemotherapy, and/or radiation therapy. While these approaches have proven successful in reducing tumor burden and destroying cancer cells, they come with harsh side effects and high chances of recurrence.
Considering these problems, other long-term strategies to treat cancer are needed. Immunotherapy is the alternative therapeutic strategy to fight cancer. Immunotherapy can be broadly defined as therapeutic measures that boost or suppress the immune responses to fight against cancer. It can either aim to directly activate the immune system to fight against the cancer cells or may augment general immune responses. Monoclonal antibody treatment, chimeric antigen receptor (CAR)-T cell therapy, and immune checkpoint inhibitors are the key immunotherapies that are being used against many cancers .
Many clinical trials are in the pipeline to investigate cancer immunotherapies’ potential . Cancer immune reprogramming can be classified in three phases:
(a) stimulation of adaptive and innate immune system to eradicate cancer cells (eradication phase),
(b) survival of irregular malignant cells which can activate immune reprogramming (equipoise phase),
(c) establishing immunosuppressive microenvironment and low-immunogenic tumors (escape phase) .
This review summarizes the different types of immunotherapies, the ongoing and/or past successful clinical trials, the current trends and research and the challenges in this field. This review will be useful for both cancer researchers and clinicians working in this direction.
Rosenberg’s Research Spans a Half-Century and Counting
About 55 years ago, Dr. Steven Rosenberg had a hunch that a patient’s own immune system could help fight cancer. Since arriving at NIH in 1974, he has painstakingly pursued this line of inquiry with some groundbreaking results.
In the beginning, his hunch diverged from all scientific literature. “It was hypothetical—no one had ever demonstrated an immune manipulation that could cause cancers to disappear,” said Rosenberg, chief of surgery at the National Cancer Institute (NCI), a position he has held for 49 years.
His research journey was inspired by two unusual cases early in his career.
In 1968, while a surgical resident at Brigham Hospital in Boston, Rosenberg was tasked with removing the gallbladder of a 63-year-old patient. The patient’s chart revealed he was hospitalized 12 years earlier with stomach cancer that had spread to the liver. Nothing could be done; no treatment was given.
“When I operated on him, he had no cancer,” recounted Rosenberg. “He had undergone one of the rarest events in all of medicine, a spontaneous regression of his metastatic cancer.”
A few years earlier, a patient at Brigham had developed widespread kidney cancer following an organ transplant. It turned out, the kidney he’d received was cancerous. When the immunosuppressive drugs—given to prevent organ rejection—were stopped, his body rejected the kidney and, incredibly, his cancer disappeared.
“That told me if you could spark a strong enough immune reaction, you could get large, vascularized cancers to disappear,” Rosenberg said. “That then set me on the path these past 50 years to try to figure out how that happened, and how to make it happen more often.”
Born and raised in the Bronx, New York, as a kid, Rosenberg wanted to be a cowboy. But that aspiration quickly turned to medicine.
Stories from his parents, Jewish immigrants who fled Poland, moved him deeply. Many of his relatives perished in the Holocaust. His family’s history instilled in him a lifelong desire to end needless suffering.
After medical school, Rosenberg paused his surgical residency to pursue a Ph.D. in biophysics at Harvard. He then conducted lab research, including a stint as an NCI immunology fellow, before finishing his residency and coming to work permanently at NCI as a clinician-scientist.
Along the way, there were false leads and failed experiments. In many cases, the cancer won.
“Most things don’t work,” as is often the plight of any clinical research, noted Rosenberg. “The overwhelming majority of experiments do not work in ways, and give the kinds of results, that we seek.”
But he persevered. “Cancer is a devastating disease. It’s a holocaust,” he said. “Patients who are innocent develop problems they cannot control, while their families sit by impotently and watch them suffer and ultimately die of the disease. It’s horrible. When you see that happening, as a doctor, you feel you have to do something about this. And that’s something that has kept me going for a very long time.”
Rosenberg ultimately became a cowboy, in a sense, exploring uncharted territory, pushing boundaries, all the while seeking to rein in cancer.
Rosenberg (l) at a weekly lab meeting (this one in 1998), where scientists, clinical fellows, technicians, students and nurses from his group convened to discuss the latest clinical and lab efforts
Rosenberg began his quest to find an immune response to cancer by studying T lymphocytes—the body’s immune warriors. But these white blood cells don’t live long outside the body. Other researchers were publishing studies with interleukin-2 (IL-2)—proteins made by white blood cells (leukocytes) that facilitate growing T cells [a type of immune cell] outside the body.
“I realized perhaps we could get anti-tumor lymphocytes to grow inside the body,” he said. “So I started administering IL-2 to treat patients.”
Rosenberg in his office with Linda Taylor in 1984. She is the first patient to respond to IL-2 administration.
It was a discouraging time, recalled Rosenberg. His team treated 66 patients in a row without success. Ongoing research showed IL-2 had a short half-life in the body of only about eight minutes. It needed to be given repeatedly in higher doses.
Then, a breakthrough. His 67th patient, Linda Taylor, received the right combination and her metastatic melanoma disappeared.
“Melanoma was previously a universally lethal disease once it had spread,” Rosenberg said. “Linda was treated in 1984 and is still alive and disease-free today.”
Rosenberg with Taylor in 2014 (r). She remains cancer-free 39 years later.
Subsequent large studies by Rosenberg’s group showed that IL-2 could induce cancer regression in 15-20% of patients with metastatic melanoma and kidney cancer. IL-2 became the first Food and Drug Administration (FDA)-approved immunotherapy for cancer in the 1990s.
Rosenberg’s team then discovered that lymphocytes that exist inside a tumor can recognize the cancer. The tumor is a sink for these immune cells, which they named “tumor-infiltrating lymphocytes” (TILs) and began administering TILs to patients.
“That was the first direct demonstration that lymphocyte transfer could cause tumor regression,” Rosenberg said. “That was developed to the point where, in our latest trials of almost 200 patients, 56% underwent a cancer regression and a quarter of patients with metastatic melanoma would undergo complete—what appeared to be durable—regressions. Now, we’re talking about response rates in the 50% range, not the 15% range of IL-2.”
Going Down Two Research Paths

CAT scans of the first patient to respond to treatment with adoptive transfer of autologous lymphocytes genetically engineered to express a chimeric antigen receptor (CAR) targeting CD19. The left side shows the lymphoma burden; at right, complete cancer regression ongoing more than 10 years later.
Building on many years of study and clinical trials, Rosenberg’s research went in two directions. One is continuing to use a patient’s own natural lymphocytes that can recognize cancer as a living drug. The second is to genetically modify immune cells to target cancer. Rosenberg was, in fact, the first person ever to insert foreign genes into a human, paving the way for the field of gene therapy.
Both types of cancer immunotherapy “are what we’re trying to improve now to fight the common solid cancers [that begin in major organs] for which there are no curable treatments, short of surgery, once they have spread.” Solid cancers account for 90% of all cancer deaths.
“It turns out, lymphocytes were recognizing the DNA mutations that cause the cancer,” distinguishing malignant cells from normal ones, Rosenberg said. “That’s probably the final common pathway of all immunotherapies…It’s somewhat ironic that the very gene mutations that cause the cancer will turn out to be its Achilles heel, to target it.
“We developed techniques to specifically study the mutation products that T cells recognized and have now developed that into a whole field of study,” he said. Initially, he focused on naturally occurring T cells.
Rosenberg (r) poses in the clinic with the first patient to respond to anti- CD19 CAR T-cell therapy, 10 years after his treatment.
When Rosenberg first proposed inserting genetically modified immune cells into patients, he not surprisingly faced major opposition—“it’s too risky” and “it’s immoral” were among the main arguments. When review board approval came, in 1984, the first patient received his own lymphocytes modified by a bacterial gene not as treatment, but to see where they went in the body.
That led his team to identify the actual lymphocyte receptors that recognize the cancer and—years later, working with Dr. James Kochenderfer, a fellow in Rosenberg’s lab—gave rise to CAR [chimeric antigen receptor] T-cell therapy—a way to modify lymphocytes to attack B-cell malignancies such as lymphoma and leukemia.
“We treated the first patient ever to receive these CAR-T cells in 2009. He underwent a complete regression and is disease-free more than 12 years later,” Rosenberg said.
Scaling Up
One of Rosenberg’s former fellows started a company to commercialize these new lab creations known as CAR-T cells, signing a cooperative research agreement with NCI. Five years later, it was sold in a multi-billion-dollar deal to Gilead Sciences.
“It’s a proud example of how findings made in government institutions can find their way out into the world,” Rosenberg said.
This process involves making retroviruses that insert and integrate appropriate genes into the patient’s normal T cells, growing them to large numbers and infusing them back into the patient. Now, NCI works with several pharmaceutical companies, with FDA approval, to develop cell transfer immunotherapies for patients with common solid cancers.
Immunotherapy can also have a negative impact on the body if it targets healthy tissue collectively known as immune related adverse events (irAE). Patients with autoimmune disease have negative effects post immunotherapy. The symptoms include from mild skin rash, headache, fatigue, joint pain to severe, affecting organ such as gut, lungs and liver as well as endocrine system . Different treatments can cause different histological iARE symptoms: for example, metastatic cancer immunotherapy that uses anti CTLA-4 leads to granulomas while anti PD-1/PDL1 generate lobular hepatitis .
Microbiota also influence potential adverse side-effects of immunotherapy such as high level of Bifidobacterium, Rhuminococcus, species of Bacteroidetes and in general greater immune diversity have positive anti-tumor effects. In contrast several factors such as microbiota driven bacterial polyamine transport, high level of serum IL-17 cytokine and tissue expressing inhibitor such as CTLA4 are negatively correlated with irAEs . As per clinical guidelines, the management of irAE depends on the grade of immunotherapy treatment as shown in Supplementary Table 5. Grade 1 to 4 is the increasing order of irAEs symptoms from mild to severe . To mitigate the adverse effects of cytotoxicity, multiple drug resistance and side effects of cancer immunotherapeutic reagents, combination therapy and nanocarrier-based delivery systems such as liposomes, nanoparticle, dendrimers and micelles can be used.
There are few challenges associated with immunotherapy. The primary one being why immunotherapy works well in some patients but not in others and how tumors that were once sensitive to immunotherapy acquire resistance. For cancer immunotherapy to be effective, one needs to find methods to manipulate the immune system of patients who fail to mount an immune response to their tumors. One way to effectively predict patient response to the immunotherapy drugs is to identify the biomarkers that can predict the patient outcome and to develop experimental models to test drug responsiveness. Besides these, there are other challenges towards developing and translating the immunotherapies such as genetic instability (e.g., heterogeneity, altered ploidy, and various mutations) in the genome of the cancer cells. This can addressed with the help of Next-generation sequencing (NGS) that allows the complete sequencing of each cell in the cancerous mass .
The use of state-of-the-art bioinformatics algorithms can help predict certain protein’s antigenicity to develop a safe and more effective personalized immunotherapy . To this effect, peptide based therapeutic vaccines have received much attention and involve CD4+T cells and CD8+T cells to target tumor associated antigens and tumor specific antigens . Clonal mutations and intra and inter tumor heterogeneity are the other challenges in personalized therapy . Usage of high throughput techniques in immunotherapy intervention such as scRNA-seq to study heterogeneity in population and to identify rare tumor populations which have altered gene expression and are resistant to killing by conventional therapies is important for the success of the immunotherapy treatment.
Research is ongoing and major advancements can be expected in the field of immunotherapy in the near future. Active research is now dedicated to exploring the role of Tregs, MDSCs, NK cells and TAMs in cancer. Further manipulation of the gut microbiome is expected to enhance the efficiency of immunotherapy. A clinical trial study of combined usage of cancer immunotherapeutic drugs along with SARS-CoV2 neutralizing antibodies provides insight for further research.
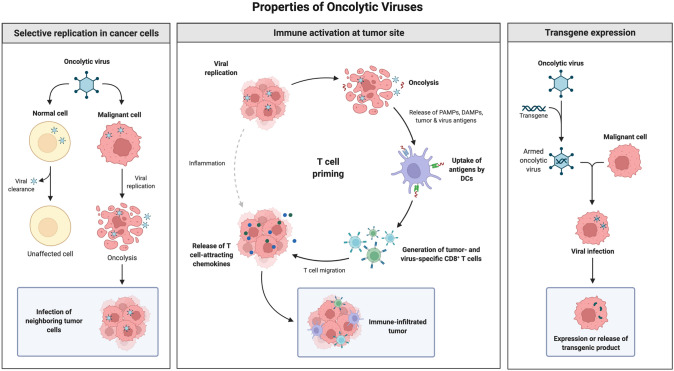
Cancer immunotherapy has emerged as one of the main pillars of cancer treatment. This is because it is personalized, targeted, and safe as compared to the other methods such as surgery, radiotherapy and chemotherapy. Immune checkpoint inhibitors such as PD-L1, PD-1 and CTLA-4 have proven to be successful in clinical trials against several cancer types. Other important immunotherapy strategies that have been sanctioned include VEGFR2, EGFR and combination strategies targeting PD-1, PD-L1, CTLA-4 and VEGFR2, EGFR. This has resulted in several FDA approved single or combination cancer immunotherapies. Alternative options of cancer immunotherapy such as CAR T cell therapy, oncolytic virus therapy, cancer vaccine provide new avenues in the direction of targeted killing tumor cells and provide better strategies to deal with toxic side effects of conventional therapy.
Creating Tomorrow’s Medicine
Rosenberg said one of the biggest challenges throughout his career has been understanding the underlying, complicated biology of cancer—the complexity of tumor recognition; identifying cells that cause cancer progression; understanding and overcoming treatment resistance.
“We study basic science and then try to translate it to patients,” he said. “That’s what NIH does. We practice the best of today’s medicine, but we’re here to create tomorrow’s medicine.”
There still is a desperate need to supplement the three existing forms of cancer treatments: surgery, radiation and chemotherapy.
“We now have this three-legged stool a lot more stable with the addition of this fourth leg,” Rosenberg concluded. “Immunotherapy has a lot of promise for developing more effective cancer treatments.”
What can you do to boost your immune system?
The idea of boosting your immunity is enticing, but the ability to do so has proved elusive for several reasons. The immune system is precisely that — a system, not a single entity. To function well, it requires balance and harmony. There is still much that researchers don’t know about the intricacies and interconnectedness of the immune response. For now, there are no scientifically proven direct links between lifestyle and enhanced immune function.
But that doesn’t mean the effects of lifestyle on the immune system aren’t intriguing and shouldn’t be studied. Researchers are exploring the effects of diet, exercise, age, psychological stress, and other factors on the immune response, both in animals and in humans. In the meantime, general healthy-living strategies make sense since they likely help immune function and they come with other proven health benefits.
Immunity in action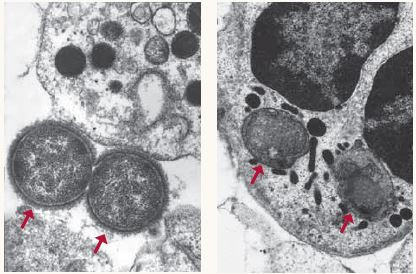 A healthy immune system can defeat invading pathogens as shown above, where two bacteria are no match for the white blood cell that engulfs and kills them (see arrows).Photos courtesy of Michael N. Starnbach, Ph.D., Harvard Medical School A healthy immune system can defeat invading pathogens as shown above, where two bacteria are no match for the white blood cell that engulfs and kills them (see arrows).Photos courtesy of Michael N. Starnbach, Ph.D., Harvard Medical School |
Healthy ways to strengthen your immune system
Your first line of defense is to choose a healthy lifestyle. Following general good-health guidelines is the single best step you can take toward naturally keeping your immune system working properly. Every part of your body, including your immune system, functions better when protected from environmental assaults and bolstered by healthy-living strategies such as these:
- Don’t smoke.
- Eat a diet high in fruits and vegetables.
- Exercise regularly.
- Maintain a healthy weight.
- If you drink alcohol, drink only in moderation.
- Get adequate sleep.
- Take steps to avoid infection, such as washing your hands frequently and cooking meats thoroughly.
- Try to minimize stress.
- Keep current with all recommended vaccines. Vaccines prime your immune system to fight off infections before they take hold in your body.
Increase immunity the healthy way
Many products on store shelves claim to boost or support immunity. But the concept of boosting immunity actually makes little sense scientifically. In fact, boosting the number of cells in your body — immune cells or others — is not necessarily a good thing. For example, athletes who engage in “blood doping” — pumping blood into their systems to boost their number of blood cells and enhance their performance — run the risk of strokes.
Attempting to boost the cells of your immune system is especially complicated because there are so many different kinds of cells in the immune system that respond to so many different microbes in so many ways. Which cells should you boost, and to what number? So far, scientists do not know the answer. What is known is that the body is continually generating immune cells. Certainly, it produces many more lymphocytes than it can possibly use. The extra cells remove themselves through a natural process of cell death called apoptosis — some before they see any action, some after the battle is won. No one knows how many cells or what the best mix of cells the immune system needs to function at its optimum level.
Immune system and age
As we age, our immune response capability becomes reduced, which in turn contributes to more infections and more cancer. As life expectancy in developed countries has increased, so too has the incidence of age-related conditions.
While some people age healthily, the conclusion of many studies is that, compared with younger people, the elderly are more likely to contract infectious diseases and, even more importantly, more likely to die from them. Respiratory infections, including, influenza, the COVID-19 virus, and particularly pneumonia are leading causes of death in people over 65 worldwide. No one knows for sure why this happens, but some scientists observe that this increased risk correlates with a decrease in T cells, possibly from the thymus atrophying with age and producing fewer T cells to fight off infection. Whether this decrease in thymus function explains the drop in T cells or whether other changes play a role is not fully understood. Others are interested in whether the bone marrow becomes less efficient at producing the stem cells that give rise to the cells of the immune system.
A reduction in immune response to infections has been demonstrated by older people’s response to vaccines. For example, studies of influenza vaccines have shown that for people over age 65, the vaccine is less effective compared to healthy children (over age 2). But despite the reduction in efficacy, vaccinations for influenza, COVID-19 and S. pneumoniae have significantly lowered the rates of sickness and death in older people when compared with no vaccination.
There appears to be a connection between nutrition and immunity in the elderly. A form of malnutrition that is surprisingly common even in affluent countries is known as “micronutrient malnutrition.” Micronutrient malnutrition, in which a person is deficient in some essential vitamins and trace minerals that are obtained from or supplemented by diet, can happen in the elderly. Older people tend to eat less and often have less variety in their diets. One important question is whether dietary supplements may help certain people maintain a healthier immune system.
Diet and your immune system
Like any fighting force, the immune system army marches on its stomach. Healthy immune system warriors need good, regular nourishment. Scientists have long recognized that people who are malnourished are more vulnerable to infectious diseases. For example, researchers don’t know whether any particular dietary factors, such as processed foods or high simple sugar intake, adversely affects immune function.
There is some experimental evidence that various micronutrient deficiencies — for example, deficiencies of zinc, selenium, iron, copper, folic acid, and vitamins A, B6, C, and E — alter cellular immune responses. However, whether that translates to changes in the human immune system and impacts on health remain unknown.
So, what can you do? If you suspect your diet is not providing you with all your micronutrient needs — maybe, for instance, you don’t like vegetables — taking a daily multivitamin and mineral supplement may bring other health benefits, beyond any possibly beneficial effects on the immune system. Taking megadoses of a single vitamin does not. More is not necessarily better.
Improve immunity with herbs and supplements?
Walk into a store, and you will find bottles of pills and herbal preparations that claim to “support immunity” or otherwise boost the health of your immune system. Although some preparations have been found to alter some components of immune function, thus far there is no evidence that they actually bolster immunity to the point where you are better protected against infection and disease. Demonstrating whether an herb — or any substance, for that matter — can enhance immunity is, as yet, a highly complicated matter. Scientists don’t know, for example, whether an herb that seems to raise the levels of antibodies in the blood is actually doing anything beneficial for overall immunity.
Does being cold give you a weak immune system?
Almost every mother has said it: “Wear a jacket or you’ll catch a cold!” Is she right? Probably not, exposure to moderate cold temperatures doesn’t increase your susceptibility to infection. There are two reasons why winter is “cold and flu season.” In the winter, people spend more time indoors, in closer contact with other people who can pass on their germs. Also the influenza virus stays airborne longer when air is cold and less humid.
A group of Canadian researchers that has reviewed hundreds of medical studies on the subject and conducted some of its own research concludes that there’s no need to worry about moderate cold exposure — it appears to have no detrimental effect on the human immune system. Should you bundle up when it’s cold outside? The answer is “yes” if you’re uncomfortable, or if you’re going to be outdoors for an extended period where such problems as frostbite and hypothermia are a risk. But don’t worry about immunity.
Exercise: Good or bad for immunity?
Regular exercise is one of the pillars of healthy living. It improves cardiovascular health, lowers blood pressure, helps control body weight, and protects against a variety of diseases. But does it help to boost your immune system naturally and keep it healthy? Just like a healthy diet, exercise can contribute to general good health and therefore to a healthy immune system.
Books can be your best pre-collapse investment.
Carnivore’s Bible (is a wellknown meat processor providing custom meat processing services locally andacross the state of Montana and more. Whether your needs are for domestic meator wild game meat processing)
The Lost Book of Remedies PDF ( contains a series of medicinal andherbal recipes to make home made remedies from medicinal plants and herbs.Chromic diseases and maladies can be overcome by taking the remediesoutlined in this book. The writer claims that his grandfather was taughtherbalism and healing whilst in active service during world war twoand that he has treated many soldiers with his home made cures. )
Easy Cellar(Info about building and managing your root cellar, plus printable plans. The book on building and using root cellars – The Complete Root Cellar Book.)
The Lost Ways (Learn the long forgotten secrets that helped our forefathers survive famines,wars,economic crisis and anything else life threw at them)
LOST WAYS 2 ( Wordof the day: Prepare! And do it the old fashion way, like our fore-fathers did it and succeed longbefore us,because what lies ahead of us will require all the help we can get. Watch this video and learn the 3 skills that ensured our ancestors survival in hard times offamine and war.)